Knowledge base
THE IMPORTANCE OF PH IN AGRICULTURE AND FERTILIZATION: TREATMENTS AND IRRIGATION
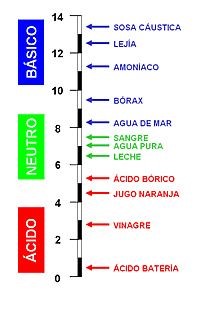
The pH is a measure of the acidity or alkalinity of an aqueous solution, with a variable value between 0 and 14. Below a pH value of 7, solutions are acidic, and above a pH value of 7, they are basic. A pH value of 7 indicates a neutral solution, which is associated with the pH of pure water. Knowledge of the pH value is crucial in numerous stages of agriculture. As a general rule, the optimal pH for agricultural applications should be slightly acidic, between pH 5-6.
pH and its control are of enormous importance in the
application of phytosanitary treatments: foliar fertilization, copper
treatment, insecticides, fungicides, herbicides, water use for irrigation, and
fertilization. In agriculture, water is essential. Its origin is diverse and
can vary depending on the time of year. Knowing its pH is important because it
can affect our harvest. It is a parameter that must be taken into account,
especially when applying phytosanitary treatments (herbicides, copper, etc.)
and in localized irrigation. A pH above six can cause products to react with
each other or with the salts in the water, leading to the creation of insoluble
substances or reduced effectiveness of the active ingredient in the
phytosanitary product.
Therefore, measuring and controlling pH can improve
treatment efficiency and save costs. It is essential to consider that the pH of
water can vary in a well within the same year and, in surface waters (canals,
reservoirs, ditches), it also exhibits variations. It is necessary to emphasize
that, in case of having a very basic or alkaline pH, it is advisable to use
specific products to lower it (acids) before mixing phytosanitary products.
Additionally, nitric acid can be used, which also provides nitrogen.
The market offers what they call pH correctors or
regulators, which are actually common acids with fancy names and high prices.
We recommend that if you want to lower the pH, a simple strong acid like nitric
acid (€0.4/kg in 2020, 63% concentration, provides 15% nitrogen) is sufficient
to regulate pH at a very low cost.
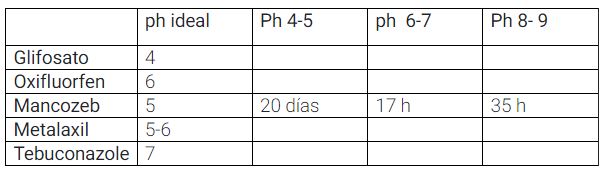
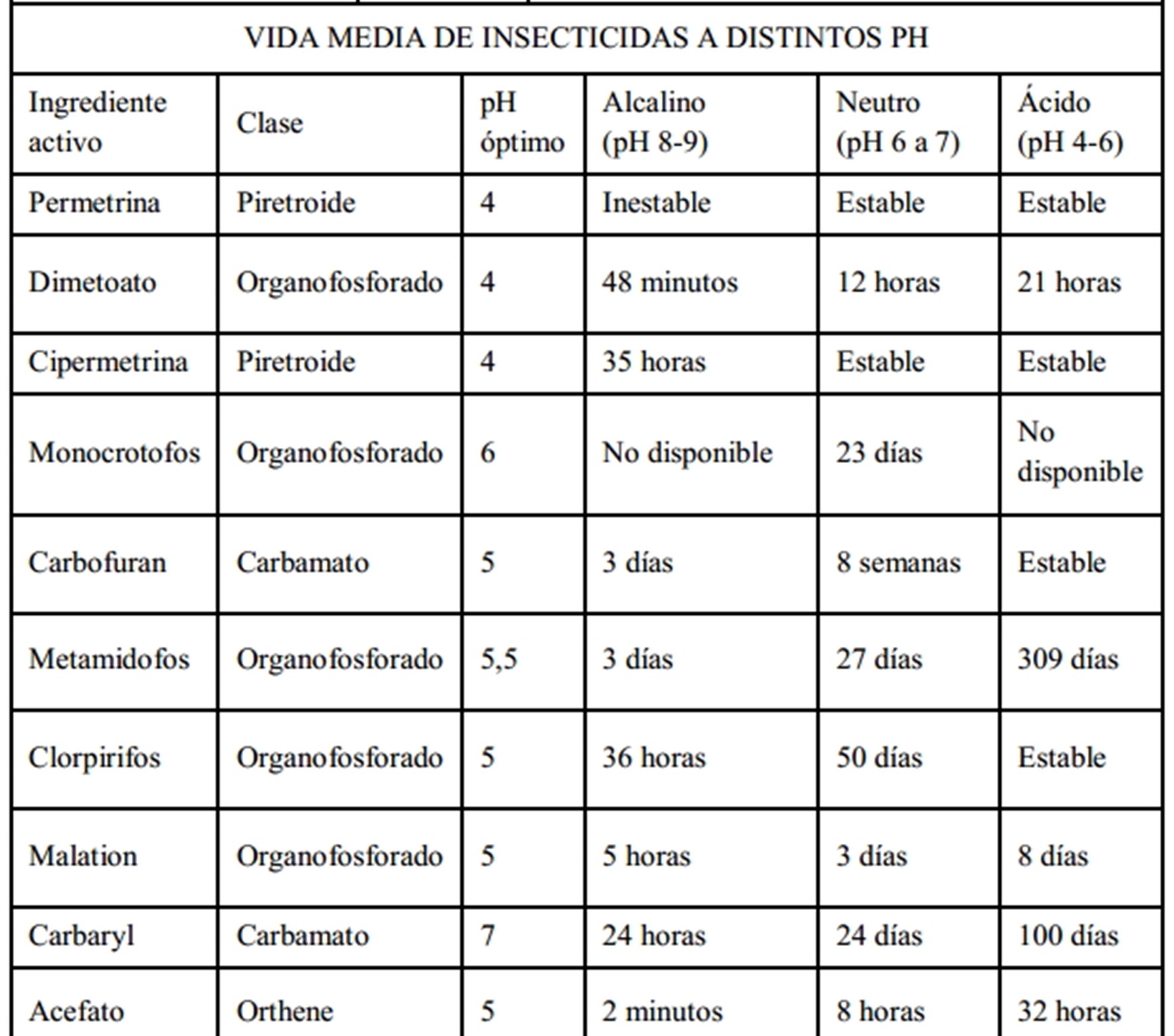
pH of the phytosanitary treatment solution
The pH determines the shelf life of the active ingredient
used. Therefore, we recommend applying it gradually and noting the quantities
used for subsequent additions during the same day. This is indicative for both
raising and lowering the pH. Thus, spending five minutes regulating the pH the
first time the phytosanitary solution is prepared can make the same treatment
highly effective or significantly reduce its efficacy. Additionally, it can be
applied later in the same day, indicating the same dosage; only the pH needs to
be tested. As mentioned earlier, a good pH regulator is nitric acid. In the
following tables, we can observe how pH influences treatment efficacy. Although
there are many variations, in general, we find that insecticides are more severely
affected than herbicides and fungicides. Furthermore, we observe that
organophosphates and carbamates decompose much faster than chlorinated
compounds.
Optimal pH and half-life of phytosanitary treatment solutions.
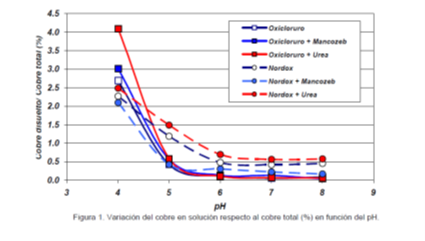
Solubility of copper compounds used in phytosanitary
treatments (Ref.: Inía - Franco Bologna and colleagues)
Copper-based compounds, in their various formulations, have
been and will continue to be one of the most commonly used tools in controlling
fungal diseases of olive trees (such as peacock spot, olive sooty mold, and
warts). The content of dissolved copper in the solution is affected by the pH
(Fig-1). The concentration of dissolved copper varies with the copper source
and the accompanying compounds in the solution. Thus, it is important to
emphasize that the lower the pH of the solution, the higher the concentration
of free copper in it. The amount of free copper at pH 4 is 50 times greater
than at pH 7 for the oxychloride solution and 5.3 times greater for the cuprous
oxide solution.
Conclusions
pH is an important factor to consider when preparing copper
solutions. At pH 6 or lower, the amount of copper provided is significantly
higher compared to the amount of soluble copper at pH 7. Variations in free
copper in the phytosanitary solution are crucial from the perspective of
treatment efficiency. The pH of the solution affects the amount of solubilized
copper, whether using copper oxychloride (Fanavid 85) or cuprous oxide (Nordox
75). The lower the pH, the higher the solubility of Cu.
With all this data, we observe the importance of pH control
in treatment solutions. Therefore, measuring pH can save a lot of costs.
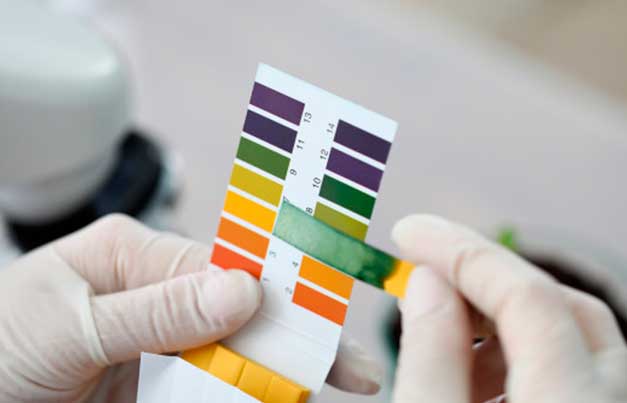
How is pH measured?
There are several ways to measure the pH of water or a
solution (treatment solution) for farmers. The simplest, easiest, and most
cost-effective method is using pH indicator strips, also known as 'Litmus
paper'. It is a quick and easy measurement system, although its accuracy may
not be comparable to a laboratory pH meter, it still provides a good low-cost solution
for farmers.
Using pH strips is straightforward. Simply dip the indicator
paper strips into the treatment solution and observe the color change after a
minute to determine whether the pH is within the desired range or if
adjustments are needed. These pH strips can be obtained for a cost of 3-5 €,
and a single package contains samples for numerous analyses.
The correct usage of the pH strip is crucial. It should be
done with clean and dry hands to avoid contamination. Spending 5 minutes to
regulate the pH during the first preparation of the phytosanitary solution can
significantly enhance treatment effectiveness or reduce its efficacy.
Furthermore, the solution can be applied later in the same day with the same
dosage; only the pH needs to be tested.
What is soil pH?
It is well-known that the choice of crop depends on soil pH.
Hence, we often say that "lupines are typical of acidic soils" or
that "alfalfa and olive prefer alkaline soils". Therefore, it is of
utmost importance to know the soil pH. The solubility of numerous
phosphorus-containing compounds in the soil is mainly determined by pH. As a
result, the pH of the nutrient solution in contact with the roots can affect
plant growth in two main ways:
- Soil
pH can affect nutrient availability. For the root system to absorb
different nutrients, they must be dissolved. Extreme pH values can lead to
the precipitation of certain nutrients, rendering them unavailable to
plants.
- pH
can affect the physiological process of nutrient absorption by the roots.
All plant species have characteristic pH parameters in which nutrient
absorption is optimal.
What is the pH of localized irrigation water (drip
irrigation)?
We know that some waters can have a high content of
carbonate or bicarbonate, known as alkaline waters. Using these waters under
certain irrigation methods (such as sprinkler irrigation) can cause significant
problems if not properly acidified beforehand (clogging of drip emitters). The
pH of the water used should be between five and six (acidic pH). Therefore, if
necessary, pH correctors (acids) should be used.
What is the pH of bottom fertilizers?
There are fertilizers that, when dissolved in water and used
as foliar fertilizers or in fertigation (MAP, MKP, phosphoric acid, nitric
acid, and ammonium sulfate), tend to acidify the treatment or irrigation water.
On the other hand, others (potassium nitrate, calcium nitrate, magnesium
nitrate, and potassium sulfate) tend to alkalize it.
From a practical point of view, and concerning pH, nutrient
solutions for fertigation can be classified into three categories:
- Optimal
(5.5 < pH ≤ 6.5)
- Suboptimal
(6.5 < pH ≤ 7.5)
- Inadequate
(pH > 7.5).
Just as the pH of water influences the preparation of the
solution, the pH of the soil affects the degradation of the products applied.
This same criterion can be used for the pH of the phytosanitary or foliar
fertilizer treatment solution.